
“Breathing technology that makes it easier to breathe. "
Endospring™ Endobronchial Coil and Delivery Catheter System:
A Revolutionary Device Offering Better Results
- Minimally invasive
- Independent of collateral flow
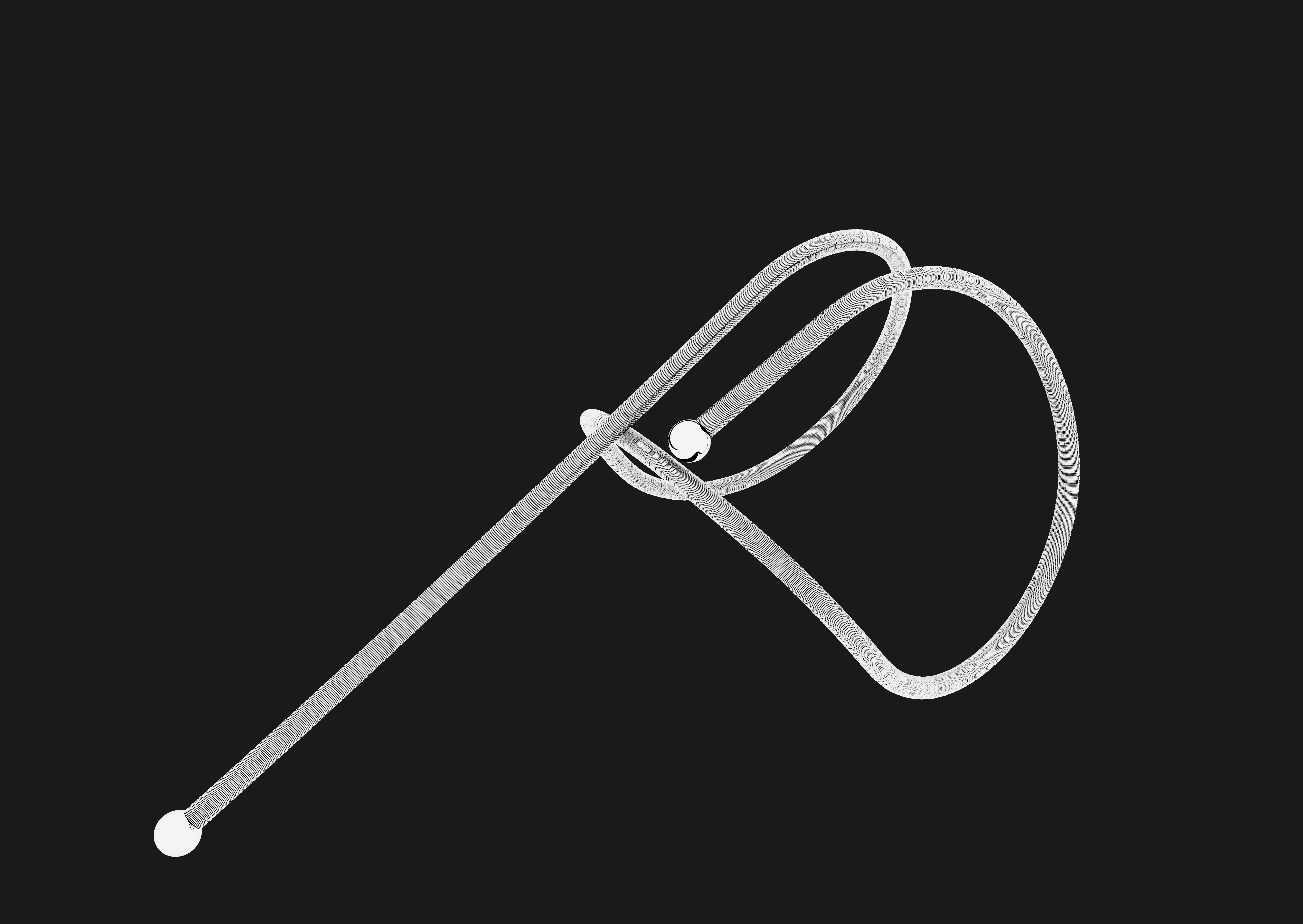
Mechanism of Action
Endospring™ Endobronchial Coil is a single-use pre-shaped nitinol implant. It is pre-installed on the tip of the delivery rod before guiding through the bronchoscope channel and then is inserted into the Endospring™ Delivery Catheter System. Endospring™ Endobronchial Coil is implanted in the target bronchus using the Endospring™ Delivery Catheter System. Endospring™ Delivery Catheter System is guided to the targeted bronchus by inserting it through the biopsy channel of the bronchoscope.Coil Then the coil is released into target destination. The delivery system is withdrawn together with the bronchoscope when it is confirmed by the direct view and the operation is completed.
Principle of Operation
The impact of the Endospring™ Endobronchial Coil and Delivery System is that the compression of the lung parenchyma by the coils results in less hyperinflation and simultaneously better transmits the elastic recoil pressure, meaning a real lung volume reduction. Secondly, the coils reduce airflow towards the targeted segments of the lung and this consequently results in a redistribution of airflow towards healthier parts of the lung. Furthermore, a decrease in airway resistance occurs in the treated lobes.Coil Finally, the volume reduction of the emphysematous treated areas could improve lung compliance and put the diaphragm in a better condition of function with, as a consequence, an increase in driving pressure of the expiratory flows.
1) Minimally Invasive Approach
Lung transplantation and lung volume reduction surgery (LVRS) are two main surgical modalities demonstrated to improve clinical and functional outcomes. LVRS is the surgical removal of diseased portions (20–35%) of the lung parenchymal volume and aims to improve the efficiency of the remaining intercostal muscles, diaphragm, and lung structure. The Endospring™ Endobronchial Coil and Delivery Catheter System is nitinol (nickel-titanium) shape- memory devices designed to restore lung elastic recoil through the compression of diseased lung parenchyma and the shortening of the airway, thereby increasing regional radial tension.Coil The Endospring™ Endobronchial Coil and Delivery Catheter System is a powerful treatment method in which the lung volume reduction coil treatment which is a minimally invasive bronchoscopic treatment option for emphysema patients who suffer from severe hyperinflation. The treatment is aimed at a large group of patients where lung volume reduction surgery is no option, or alternatively, can be offered as a bridge to lung transplantation. Due to the high complication rate and risk of mortality observed during the National Emphysema Treatment Trial (NETT),in recent years, less invasive emphysema treatment procedures and bronchoscopic approaches are developed. These methods are proven effective and safe within the short- to the medium-term clinical trials.
2) Independent of collateral flow
Differently from one-way valves, collateral ventilation do not interfere with coils treatment outcomes. Endobronchial coils certainly appear to have a very good short-term safety profile. By applying traction forces to lung parenchyma, lung volume reduction coils aim to improve hyperinflation and gas trapping by reducing dynamic airway collapse. The mechanism of action is independent of collateral ventilation and could be applied to emphysema that is ho-mogeneous or heterogeneous.
Indications
Endospring™ Endobronchial Coil and Delivery Catheter System are minimally invasive devices designed for the treatment of both heterogeneous and homogeneous emphysema. Coil The system is indicated for use in patients with severe emphysema to improve quality of life, lung function, and exercise capacity. It is indicated for the adult patients with homogeneous and/or heterogeneous advanced emphysema and severe lung hyperinflation to control prolonged air leaks of the lung and improve and ameliorate lung functions. There is no study done for pregnant women, patients over <70yo and younger than 18yo. It is indicated when little to no collateral ventilation. It is not recommended to use at this patient group.




